
Reliance on a single IRB typically occurs with:
Decisions about whether Weill Cornell Medicine will enter into an Authorization Agreement review, whereby the WCM IRB will rely on the IRB at another institution, or will review for other institutions, are handled on a protocol-specific, case-by-case basis once those requests have been submitted. To initiate a request for IRB reliance, complete the WCM reliance request form.
An authorization agreement refers to the formal written agreement that documents respective authorities, roles, responsibilities, and communication between an institution/organization serving as the IRB of Record (Reviewing IRB) and the institution relying on that IRB (Relying IRB). This term includes: reliance agreement, cooperative agreement, master services agreement (MSA), master joint agreement (MJA), or umbrella agreement.
Researchers seeking to rely on the IRB of another institution or have the WCM IRB serve as the reviewing IRB for another institution must have an IRB authorization agreement. These agreements are executed between a Reviewing IRB and one or more Relying Institutions and delineate the roles and responsibilities of the involved parties. The agreements can be for a single research study or for multiple studies (e.g., a master reliance agreement). The authorization agreement is negotiated and finalized by the WCM IRB and reviewed by WCM's legal counsel, as needed.
Authorization agreements do not replace the need for IRB approval.
Even with an authorization agreement, researchers must still obtain IRB approval from the reviewing IRB before beginning any study activities and before funds can be released. The WCM principal investigator remains responsible for ensuring all of WCM institutional requirements are met before beginning the research and throughout the course of the research activities.
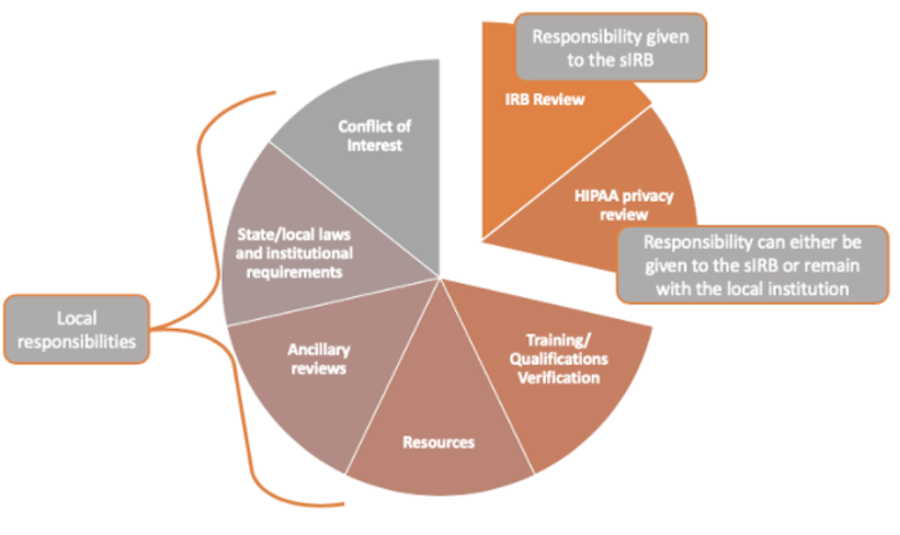
sIRB review is more than just an IRB review. It includes other components such as COI, state/local laws and institutional requirements, ancillary reviews, etc.
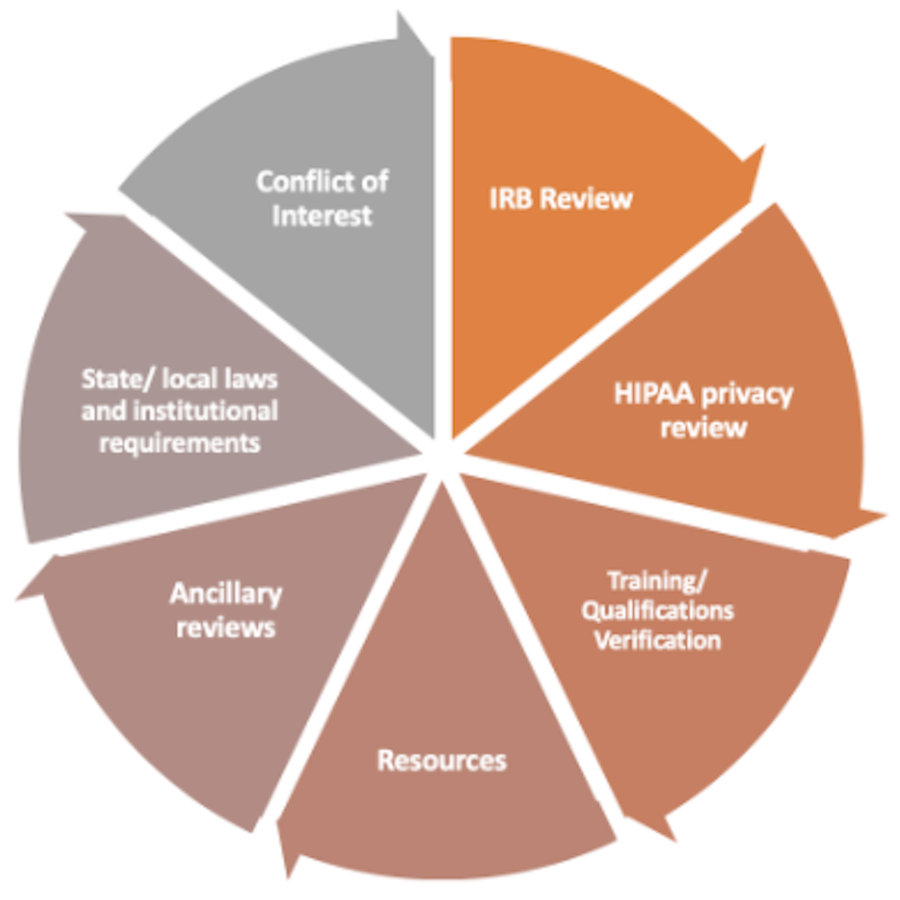
The image below depicts the different responsibilities of the sIRB compared to the local institution.
When it has been established that a trial will utilize single IRB review, a determination must be made on which campus’s or institution’s IRB will oversee the trial conduct. To facilitate this process, both the WCM and External IRBs will collect and review basic information about the planned collaboration.
The WCM Principal Investigator (PI) or designee must initiate a request for IRB reliance by completing the WCM reliance request form.
Once a request for reliance has been received, staff from the WCM and External IRB(s) will jointly determine who will serve as the IRB of record for any given collaboration. A response from the IRB office will be provided within two business days of reliance request receipt.
The determination on which institution will serve as IRB of record for collaborative human subjects research will take into consideration the following:
Industry Sponsored multisite research will utilize a commercial IRB, unless an academic IRB has been identified and is in use.
If the study has been expired for more than 90 days in WRG-HS, an automatic closure will occur.
If you wish to re-open your submission please complete the Request to Re-open Closed/Withdrawn Submission form. The completed form will be routed to our leadership team for review. Someone will reach out to you if more information is needed to support your request.