
Deciding whether or not to participate in a research study can be challenging on our own, and this is especially true if you are new to research. The following resources are provided for you to support your decision-making process, and to answer any questions you might have about what participation entails.
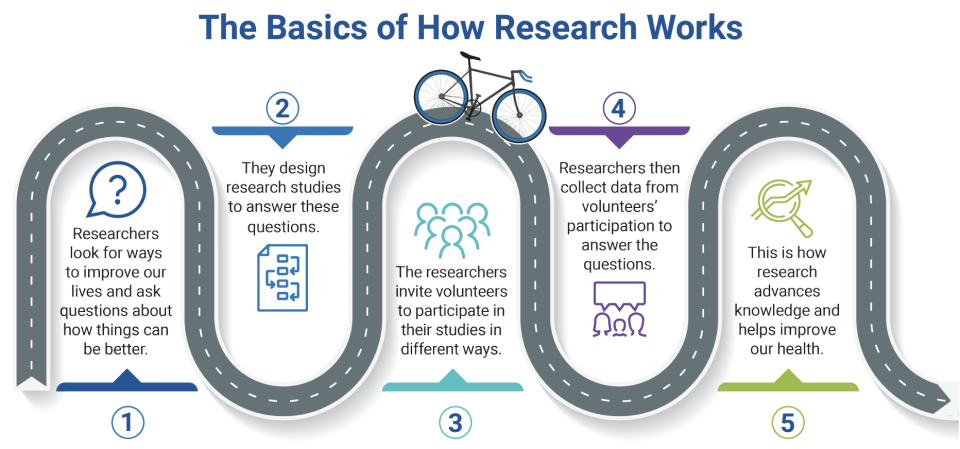
from the Office of Human Research Protections About Research Participation (ARP) pamphlet.
The Office for Human Research Protections (OHRP) is a federal government agency that is responsible for providing leadership in the protection of research subjects. The OHRP website provides a wealth of information for research volunteers:
The National Institutes of Health (NIH) is part of the U.S. Department of Health and Human Services tasked with investigating and preventing disease, and improving population health. The provide a wealth of information for patients who are considering joining a clinical trial that can be accessed on their Clinical Research Trials and You page, or by clicking links to specific pages below. The NIH also maintains a Spanish-language version of their page.