
The typical progression of a submission through the IRB application process begins when the Investigator submits an Initial Application in the WRG-HS system. That then sets off a number of processes that must be complete before IRB analyst review.
For an overview of the IRB review process at WCM, please review the recording of our recent presentation, IRB101: Introduction to the WCM IRB, available on our Human Research Compliance Monthly Education and Training Series (METS) page.
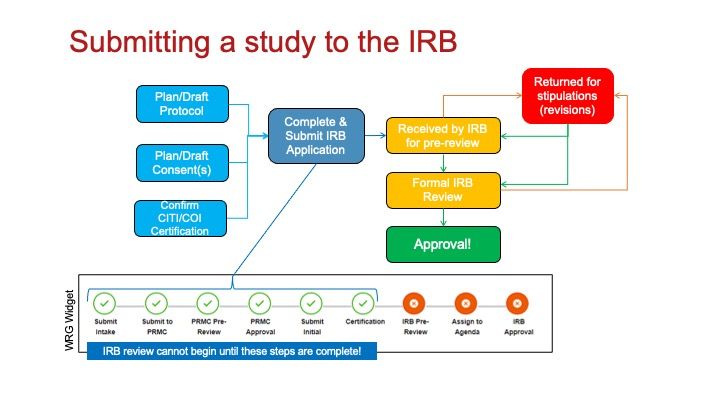
Step 1: Any investigator conducting human subjects research must apply for IRB approval. The first step in this process is to complete an Intake Form in WRG-HS.
Step 2: Once the intake form is completed, an application to the PRMC (or equivalent) must be submitted.
Step 3: Once PRMC (or equivalent) approval has been obtained, complete an initial submission in WRG-HS. Once your initial submission is submitted, several steps occur:
Step 4: Pre-review
An IRB analyst audits the application to ensure all regulatory requirements have been met.
Step 5: The complete application is assigned to an IRB agenda for review.
Step 6: The IRB member(s) review the entire application and discuss any item of concern during a convened IRB meeting., or if expedited, issues a request for modification(s) letter.
Step 7: The IRB Determination Letter is released and entered into the WRG-HS system.